Neuromyelitis optica (NMO), also known as Devic’s disease, is a rare autoimmune disorder primarily affecting the optic nerves and spinal cord.
Neuromyelitis optica (NMO), also known as Devic’s disease, is a rare autoimmune disorder primarily affecting the optic nerves and spinal cord. Think of it like your body’s immune system mistakenly attacking these vital parts of your central nervous system. The hallmark of NMO is inflammation of the optic nerve (optic neuritis), which can cause eye pain and vision loss in one or both eyes. People with NMO also experience inflammation of the spinal cord (myelitis), leading to symptoms like muscle weakness, numbness, pain, and problems with bladder and bowel control. What makes NMO distinct from multiple sclerosis (MS), another autoimmune disease of the central nervous system, is the presence of a specific antibody called anti-aquaporin-4 (AQP4) antibody in most individuals with NMO. Aquaporin-4 is a protein found in high concentrations in the optic nerves and spinal cord, and this antibody targets and damages cells expressing this protein. NMO attacks can be severe and can lead to permanent disability if not treated promptly. While there’s no cure for NMO, treatments focus on managing acute attacks, preventing relapses, and alleviating symptoms. These treatments often involve immunosuppressive therapies to reduce the abnormal immune response. Early diagnosis and appropriate management are crucial for improving the long-term outcomes for individuals living with NMO.
The Systems Architecture of NMO is published as a web based tool open to public . Click below to interact with the Systems Architecture.
A peer-reviewed paper from the NMO Initiative will be published in the Journal for the benefit of community. This phase is yet to begin.
In this phase, the NMO initiative will conduct in silico modeling to identify and test the efficacy of natural compounds against the NMO diseases. This phase is yet to begin.
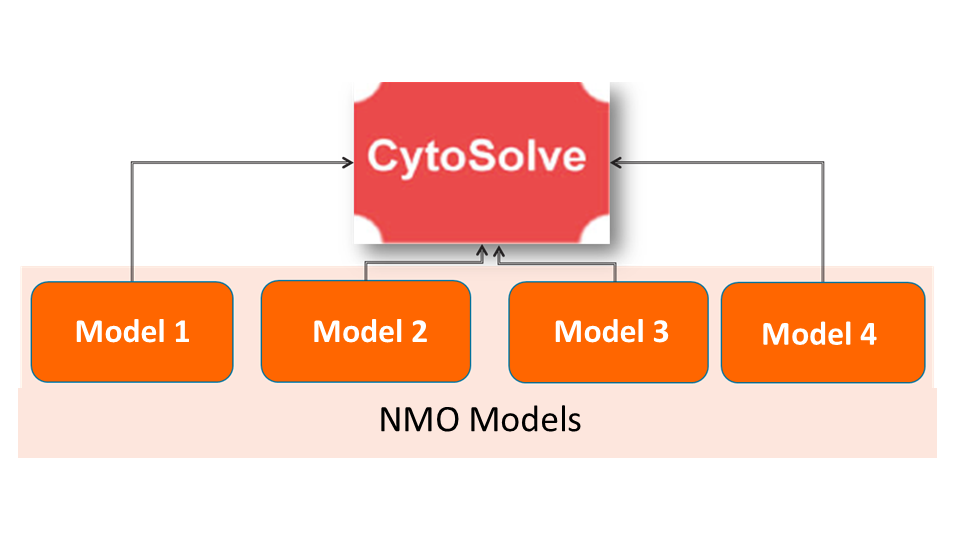
In this phase, combination screening will be performed to identify potential ingredient/compounds that target the biological process implicated in NMO pathogenesis. This phase is yet to begin.
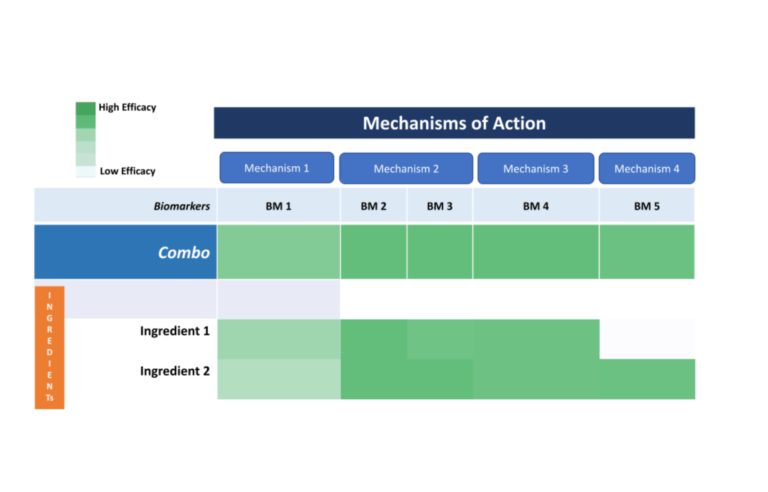
The Open Science Institute® through its NMO Initiative is moving towards getting patents for a revolutionary molecule that effectively combats periodontal disease, contributing breakthrough real solutions to society worldwide.
The NMO Initiative plans to discover, develop, license and manufacture proprietary nutraceuticals to support treatment of NMO. Support our mission to bring this innovation to those who need it most. Please support this phase by donating to the NMO Initiative.

