
Osteoarthritis is a degenerative joint disease characterized by the breakdown of cartilage, leading to pain, stiffness, and reduced mobility.
The Open Science Institute’s Osteoarthritis research project has progressed now to the sixth stage of licensing and manufacturing. Our goal is to raise $60,000 to go direct to the public including vehicles such as satellite radio and offline posters, flyers, banners. Please support this initiative.
Osteoarthritis is the most common form of arthritis, often affecting the hands, knees, hips, and spine. Management typically involves a combination of exercise, physical therapy, weight management, and pain relief medications. The Open Science Institute’s Osteoarthritis Initiative has been established to provide a comprehensive and integrative molecular systems understanding of human knee osteoarthritis (OA) and develop therapeutic interventions to address Osteoarthritis.The architecture is constructed through a supervised bioinformatics process beginning with nearly 20,000 scientific papers curated to a refined set of 5,243 papers from which molecular interactions are extracted. The systems architecture is accessed through an easy-to-use interface to traverse the complexity of knee OA systems biology from tissue to cell to an ensemble of molecular interactions whereby one may discover the particular paper from which an interaction is derived. The systems architecture maintains its currency by enabling the research community to provide feedback that can be reviewed, refereed and incorporated. The architecture provides a framework for developing new educational tools as well as to support research that aims to investigate disease mechanisms, and identify potential therapeutic targets for the treatment of knee OA.
The Systems Architecture of Osteoarthritis is published as a web based tool open to public. Click below to interact with the Systems Architecture.
A peer-reviewed publication resulting from the work of Osteoarthritis initiative was published in 2022 in the Journal of Applied Sciences. Download the paper below
In this Phase, the Osteoarthritis Initiative conducted in silico modeling to identify and test the efficacy of two bioflavonoids. The results are shown below.
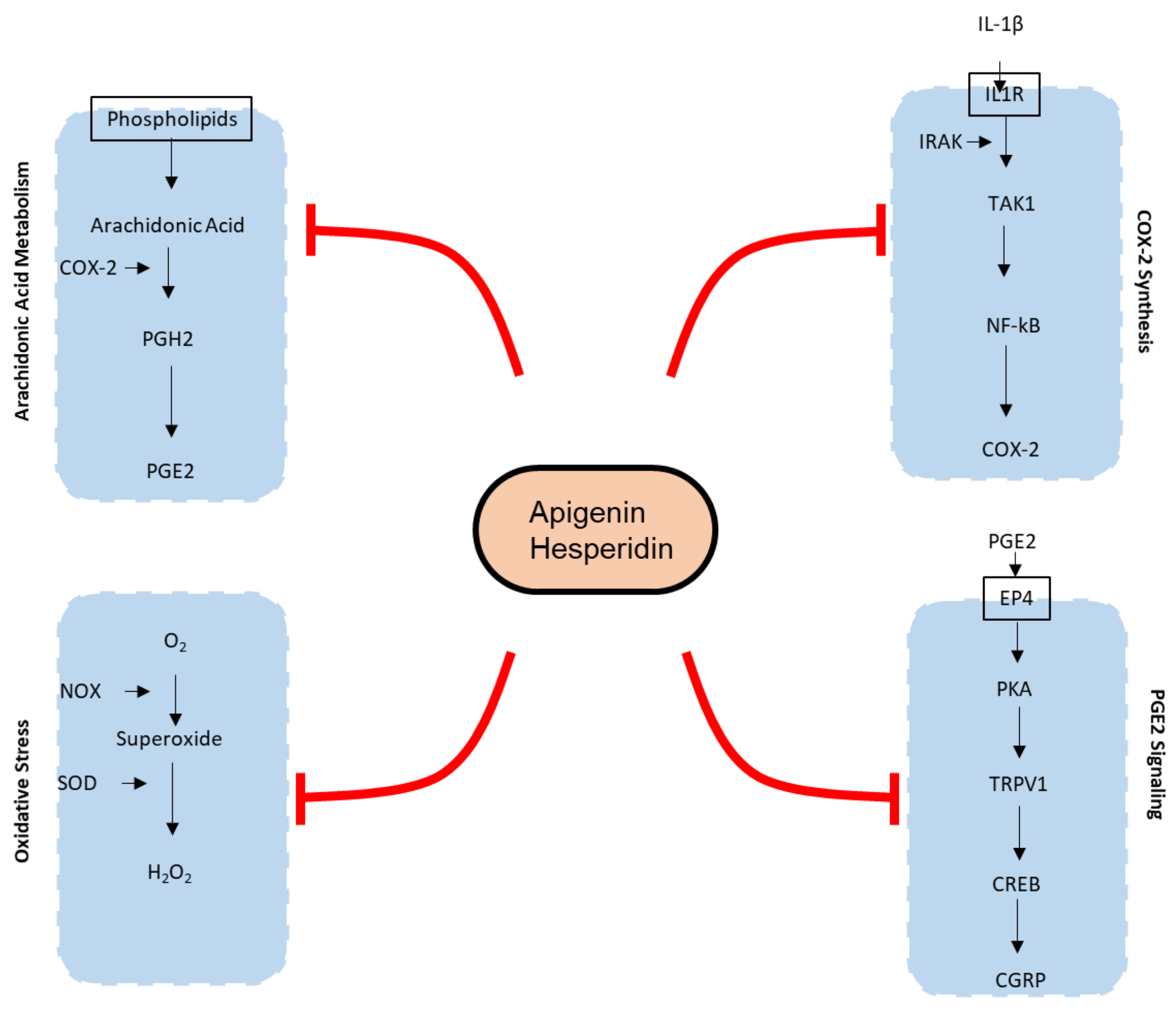
Mechanisms of action of joint pain affected by apigenin and hesperidin. Oval with black outline contains the bioactive molecules. Apigenin and hesperidin affect five biomarkers of joint pain—COX-2, PGE2, TRPV1, CGRP, and ROS—in both chondrocytes as well as endothelial cells. COX-2—Cyclooxygenase 2; PGH2—Prostaglandin H2; PGE2—Prostaglandin E2; IL-1β—Interleukin 1β; IL1R—Interleukin 1 beta receptor; TAK1—Transforming growth factor-β (TGF-β)-activated kinase 1; NFκB—Nuclear factor kappa-light-chain-enhancer of activated B cells; O2—oxygen; H2O2—hydrogen peroxide; NOX—NADPH oxidase; SOD—superoxide dismutase; PKA—protein kinase A; CREB—cAMP response element-binding protein; CGRP—calcitonin gene-related peptide.
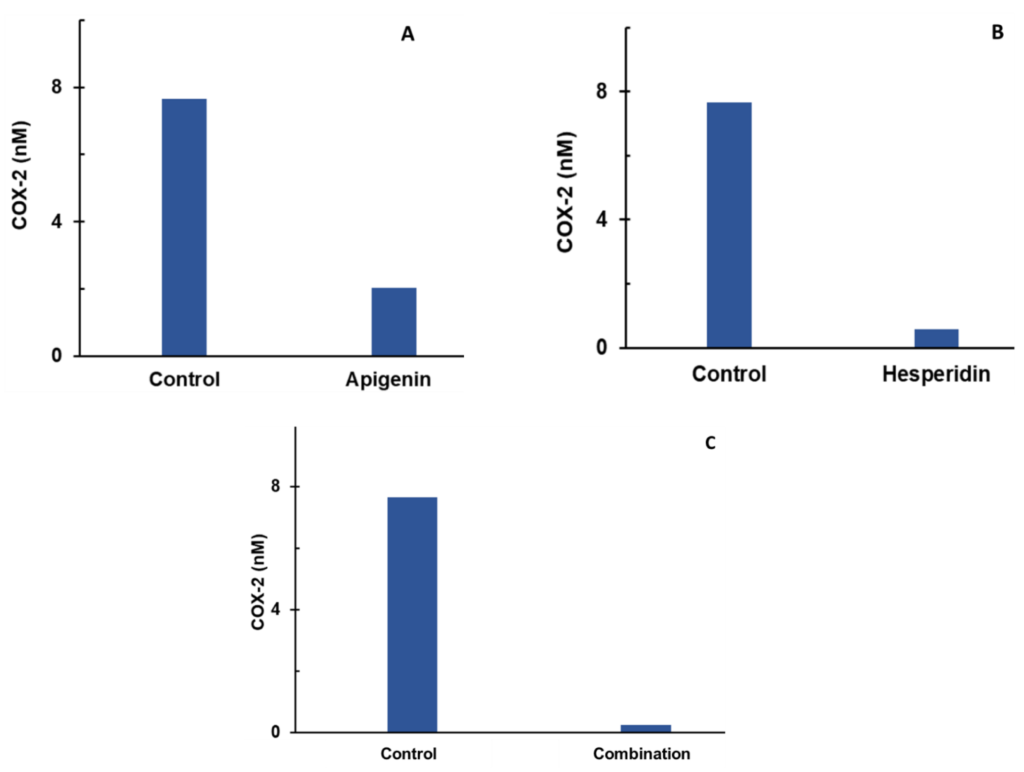
Individual (A,B) and combination effect (C) of apigenin and hesperidin on COX-2 production in chondrocytes over simulations periods of 2 days. Apigenin at 30 mg dose (A), hesperidin at 1000 mg dose, (B) and their combination at 1030 mg dose (C) reduced the levels of COX-2 over a period of 2 days. COX-2—cyclooxygenase 2.
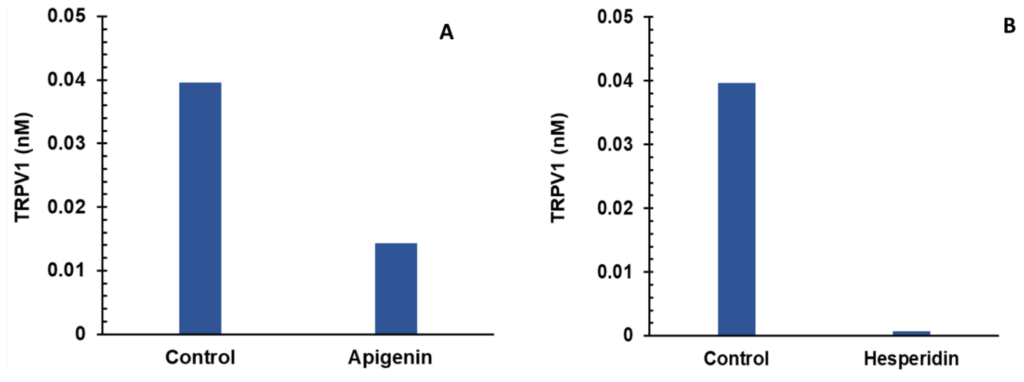
Individual (A,B) and combination effect (C) of apigenin and hesperidin on TRPV1 production in chondrocytes over simulations periods of 2 days. Apigenin at 30 mg dose (A), hesperidin at 1000 mg dose, (B) and their combination at 1030 mg dose (C) reduced the levels of TRPV1 over a period of 2 days. Individual (panels D and E) and combination effect (panel F) of apigenin and hesperidin on CGRP production in chondrocytes over simulation periods of 2 days. Apigenin at 30 mg dose (D), hesperidin at 1000 mg dose, (E) and their combination at 1030 mg dose (F) reduced the levels of CGRP over a period of 2 days. TRPV1—transient receptor potential cation channel subfamily V member 1. CGRP—calcitonin gene-related peptide.
Individual (A,B) and combination effect (C) of apigenin and hesperidin on TRPV1 production in chondrocytes over simulations periods of 2 days. Apigenin at 30 mg dose (A), hesperidin at 1000 mg dose, (B) and their combination at 1030 mg dose (C) reduced the levels of TRPV1 over a period of 2 days. Individual (panels D and E) and combination effect (panel F) of apigenin and hesperidin on CGRP production in chondrocytes over simulation periods of 2 days. Apigenin at 30 mg dose (D), hesperidin at 1000 mg dose, (E) and their combination at 1030 mg dose (F) reduced the levels of CGRP over a period of 2 days. TRPV1—transient receptor potential cation channel subfamily V member 1. CGRP—calcitonin gene-related peptide.
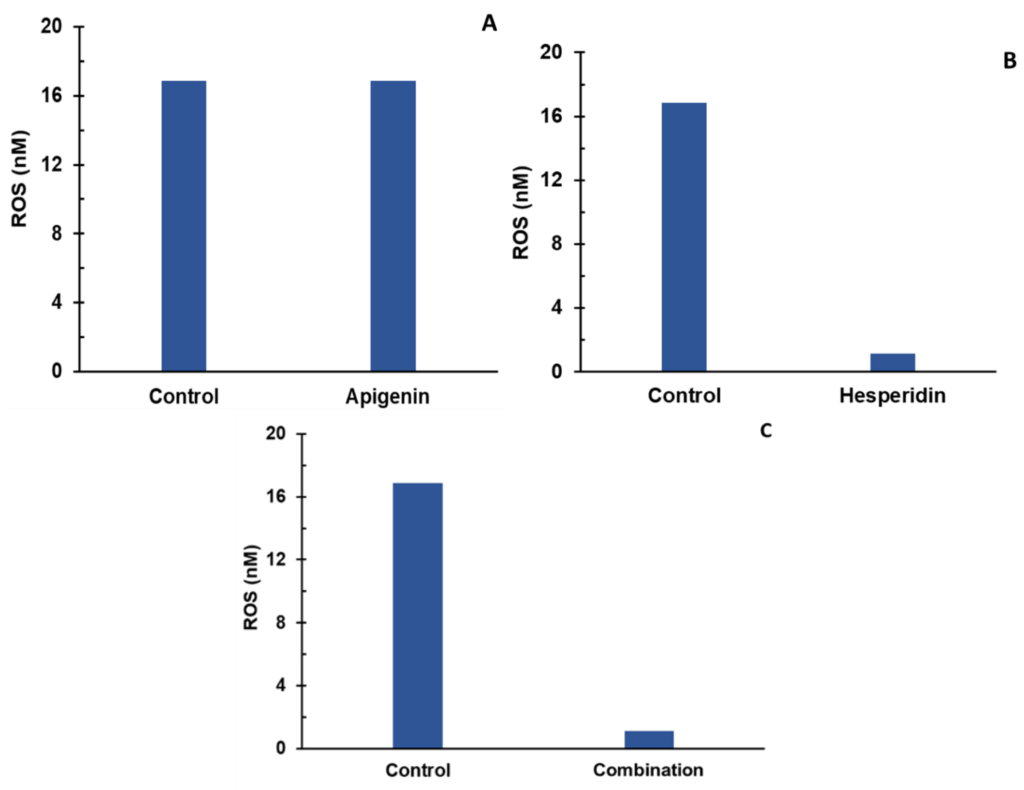
In this Phase, natural ingredients were identified and selected to be combined that had optimal effect on the biomarkers of COX-2, PGE2, TRPV1, CGRP, and ROS. The dose levels of two bioflavonoids – Apigenin and Hesperidin – were finalized in the optimal combination
The optimal combination of Apigenin and Hesperidin showed synergistic effect on four of the five biomarkers, which helped in successful patent application. The patent for this combination was granted in 2023.
The Osteoarthritis Initiative is in the “Licensing and Manufacturing” Phase. The Apigenin and Hesperidin combination is branded as “mV25™ – Momentum to moVe” and is available for purchase. Please support this Phase by donating to the Osteoarthritis Initiative, signing up to become distributor of mV25™, or purchasing mV25™.
