PEER-REVIEWED PAPER SUGGESTS GENETICALLY ENGINEERED SOY (GMO) PRODUCES
EXCESS FORMALDEHYDE AND DISRUPTS NATURAL PLANT METABOLISM
A new study published in the peer-reviewed journal AGRICULTURAL SCIENCES applies modern computational systems biology methods to reveal genetically engineered soy (the GMO) creates significant disruption to the levels of formaldehyde, a known carcinogen, and glutathione, an important anti-oxidant necessary for cellular detoxification.
In the GMO, formaldehyde dramatically accumulates (Illustration 1) and glutathione is depleted (Illustration 2).
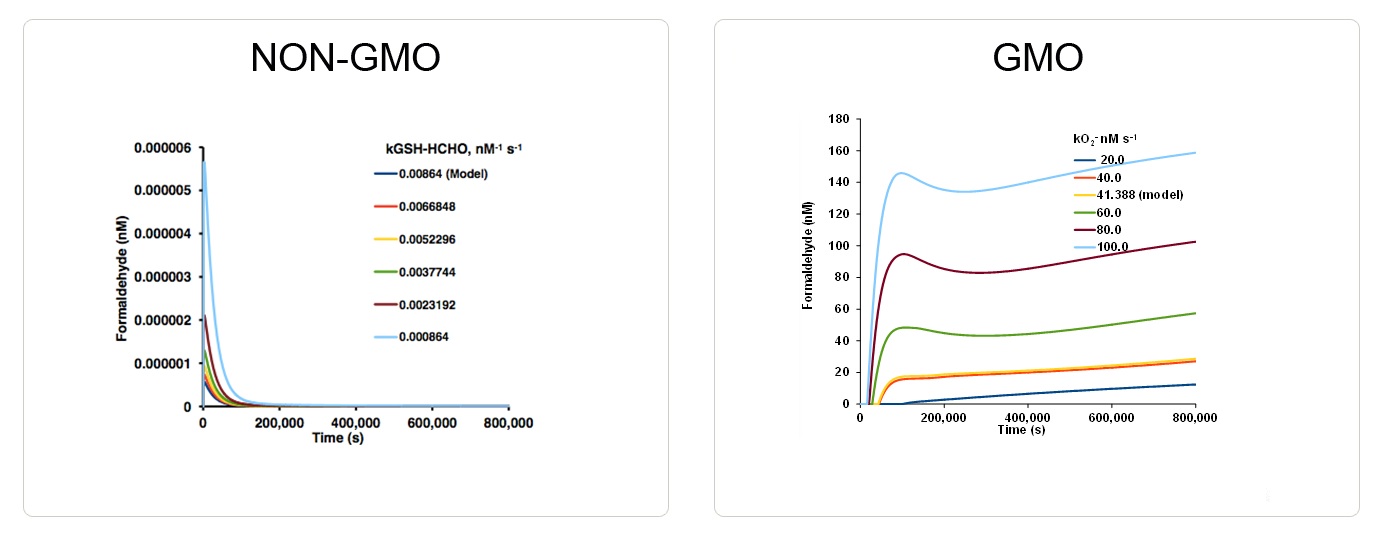
Illustration 1: Formaldehyde Accumulation in GMO
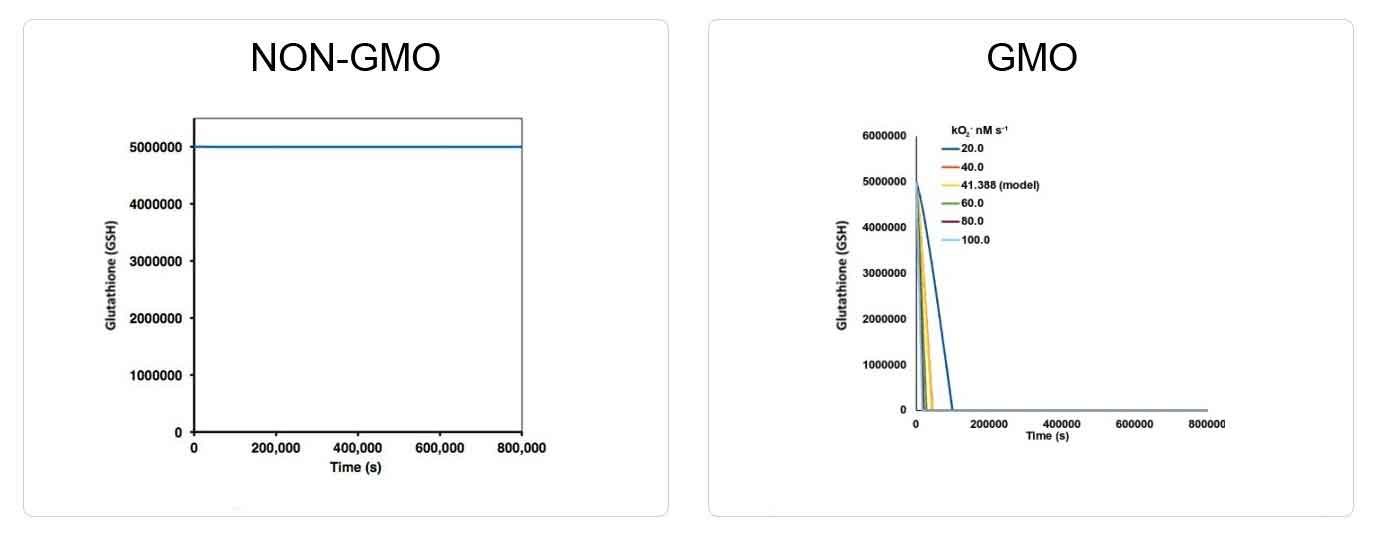
Illustration 2: Glutathione Depletion in GMO
The study is the first systems biology analysis of its kind and is an outcome of three previous scientific papers, published in AGRICULTURAL SCIENCES and THE AMERICAN JOURNAL OF PLANT SCIENCES.
The U.S. government’s gold standard for safety assessment of GMOs is based on the principle of “substantial equivalence,” which deems a GMO safe for human consumption and allows it to be fast-tracked to market, without any real testing, if the GMO, based on certain criteria, is “substantially equivalent” to its non-GMO counterpart. The study suggests that if formaldehyde and glutathione were used as criteria for assessment, then the GMO would not be “equivalent” to its non-GMO counterpart, and would not have been allowed.
Dr. V.A. Shiva Ayyadurai, Ph.D., an MIT-trained scientist and systems biologist, who is widely recognized as the inventor of email, is the lead scientist on the study. Dr. Ayyadurai believes that this study provides a new paradigm to address the safety of GMO’s by developing transparent Industry Standards for real testing of GMOs, while employing computational systems biology methods to identify real and relevant criteria, to support such testing.
The publication of the paper coincides with release of a bulletin by the Obama Administration on July 2, 2015, calling for “Improving Transparency and Ensuring Continued Safety in Biotechnology.” Scientists and physicians are supportive of the recent study, formally being signatories of an official statement (see below), and believe that the results are substantive enough to conclude that it is premature to approve GMOs until such Standards are created.
Ayyadurai shares, “This is not a pro- or anti-GMO question. But, are we following the scientific method to ensure the safety of our food supply? Right now, the answer is ‘no.’ We need to, and we can if we engage in open, transparent and collaborative scientific discourse, based on a systems approach.”




